Amorphous Content Determination -
One significant benefit of DVS is its ability to detect low levels of amorphous content in pharmaceutical powders. Amorphous content in pharmaceutical materials can lead to instability and reduced efficacy due to their tendency to absorb more moisture, causing phase transitions and product degradation. The DVS is an effective method to quantify amorphous content by analyzing moisture sorption isotherms. It allows scientists to calibrate known amorphous contents or identify vapors that induce crystallization, thereby understanding the extent of amorphous phases The example shown presents data where crystallization of a pharmaceutical ingredient was induced using ethanol as a solvent (Figure 1). After establishing a baseline, the system was adjusted to a low partial pressure environment before initiating crystallization at 30% p/p0. To induce a phase change, ethanol concentration was then increased to 85% p/p0, resulting in the formation of a crystalline material. Due to the formation of a structured crystalline lattice, there was a reduced uptake of ethanol when the system returned to 30% p/p0 in the third stage of the experiment. The amorphous content was calculated based on the difference in ethanol uptake between the first and third steps of the experiment. Such studies enhance pharmaceutical stability and shelf life by identifying and controlling unstable amorphous regions.
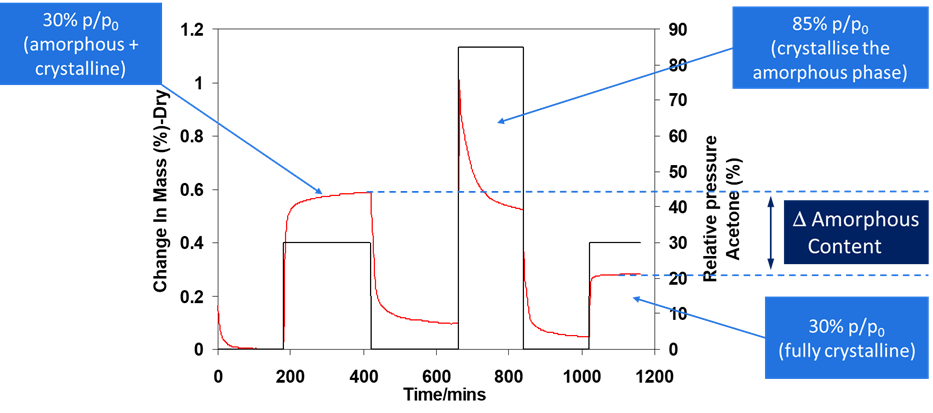 Figure 1. The determination of amorphous content of a
pharmaceutical material using the DVS
Figure 1. The determination of amorphous content of a
pharmaceutical material using the DVS
Analysis of Drug-Excipients Moisture Interactions -
Moisture sorption isotherms, which describe the equilibrium moisture content of a material at different relative humidity levels, are essential for understanding and predicting the behavior of pharmaceuticals under various environmental conditions. DVS allows for precise measurement of these isotherms, providing detailed insights into the hygroscopicity of both active pharmaceutical ingredients (APIs) and excipients. This information is crucial for designing stable and effective formulations. For example, by analyzing the moisture sorption properties of hydrophobic pharmaceutical substances. Figure 2 displays the water sorption isotherms for the individual components of hydroxypropyl cellulose (HPC), microcrystalline cellulose (MCC) and Lovastatin mixture. The percent change in mass (referenced to the dry mass) is plotted on the y-axis, while the target relative humidity is plotted on the x-axis. Lovastatin is very hydrophobic, as indicated by the minimal percentage change in mass between 0 and 95% RH. HPC and MCC are significantly more hydrophilic. The DVS can reveal how these materials interact with moisture which helps in selecting suitable excipients and optimizing formulations to enhance drug performance and shelf life
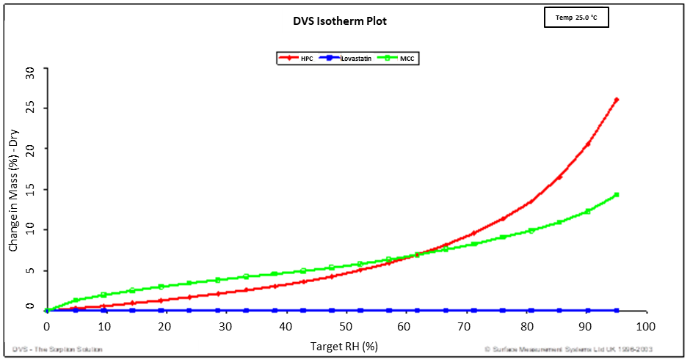
Figure 2. Water sorption isotherms for HPC (red), MCC (green), and Lovastatin (blue) 25.0°C
Determination of Glass Transition RH -
The DVS can determine the critical relative humidity (RH) at which a glass transition occurs. This is important because the glass transition can lead to significant changes in a material’s physical properties. Figure 3 shows a DVS ramping experiment coupled with a camera to analyse spray dried lactose. The experiment starts with a stable baseline, then increases the relative humidity by 10% per hour up to 90%. Initially, moisture adsorbs on the surface. As RH increases, lactose undergoes a glass transition, where moisture penetrates the bulk, increasing molecular mobility and facilitating recrystallization above 80% RH. This absorbed moisture acts as a plasticizer, promoting the rearrangement of lactose molecules into a stable crystalline form. Further increases in RH lead to material decomposition. In identifying the glass transition RH, manufacturers can better understand and control the storage and processing conditions of amorphous pharmaceuticals.
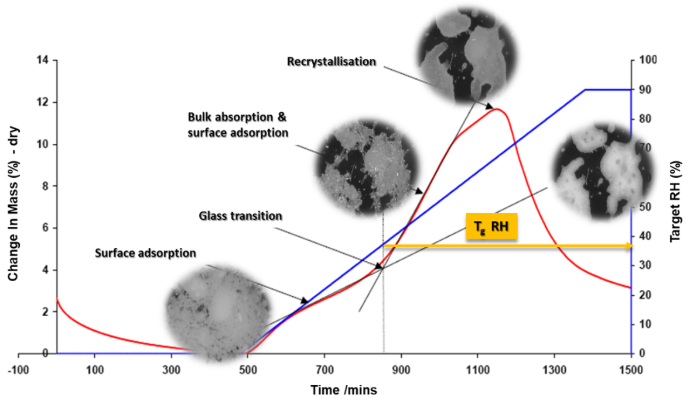
Figure 3. Ramping experiment of spray dried lactose from 0-90% RH at a rate of 10% RH per hour showing a phase change through the glass transition stage with sample photos at each stage
Packaging Materials -
Packaging materials play a crucial role in protecting food products from environmental factors, and their ability to interact with moisture can significantly impact the shelf life and quality of the packaged goods. The DVS can be used to understand how packaging materials absorb and desorb moisture under varying humidity conditions, providing insights into their water-vapor transmission rates and barrier properties. By exposing packaging samples to controlled humidity levels and continuously measuring the weight changes, the DVS can determine moisture diffusion coefficients. This information is important for selecting appropriate packaging materials that maintain product stability and prevent issues such as spoilage, caking, or loss of flavour and aroma. Moreover, DVS can identify critical humidity thresholds that might induce phase transitions or degradation in packaging materials, allowing manufacturers to design more effective packaging solutions that ensure longer shelf life and better preservation of food products.

A laboratory process showing a person in gloves carefully sifting a white powdered substance using a fine mesh sieve, likely for scientific or pharmaceutical purposes."
Improvement in Quality Control and Assurance -
DVS serves as a powerful tool for quality control in pharmaceutical manufacturing. It ensures that materials meet the required specifications for moisture content, which is crucial for maintaining product quality and compliance with regulatory standards. Regular use of DVS in quality assurance processes ensures that each batch of the product is consistent with the intended specifications.
Dynamic Vapor Sorption (DVS) is an indispensable tool in the pharmaceutical industry, offering enhanced sensitivity, detailed moisture sorption analysis, and valuable insights into drug-excipient interactions. Its ability to detect low levels of amorphous content, study complex formulations, and ensure product stability and quality makes it a crucial technique for pharmaceutical research, development, and manufacturing. In the research and development phase, the DVS accelerates the formulation process by providing quick and accurate data on the moisture-related properties of new compounds and formulations. This enables researchers to make informed decisions early in the development process, reducing the time and cost associated with bringing new drugs to market. As the industry continues to evolve, the role of DVS in ensuring the efficacy and safety of pharmaceutical products will only become more prominent, driving innovation, and improving patient outcomes.
